
Behavior of chemical
Human health effects of hazardous chemicals
Response exposure limits
NIOSH pocketguide
Level A
Level B
Level C
Level D
Chemical protective cloths
Site control
EPA suggested Decontamination line
Hazard identification
There are many terms that used to identify chemicals. Most chemicals were classified by the method of listing of federal agencies; OSHA, EPA, DOT.
OSHA defined the chemicals as hazardous chemicals that might
have potential hazards to human health. The terms of classification are on
purpose to protect human health. The regulation enforced producer or manufacture
to comply with safe environment of employees and people in community. In 29 CFR
1910.1200 Hazardcom, requires manufacturer to prepare Materials Safety Data
Sheet (MSDS) to provide information
about the hazards of the chemicals in operating or handling activity.
EPA defined chemicals in the term of hazardous chemicals
that can present danger to the environment. The report must be send to EPA in
case of chemicals spill exceeding the report quantity on the purpose to protect
air, ground and water resources. EPA also regulated the Hazardous waste
under the Resource Conservation and Recovery ct 1976 RCRA to institute
waste management program initiating “cradle to grave” for tracking of
hazardous waste.
DOT defined term of hazardous materials (dangerous goods)
that may present danger during transportation by truck, rail, air, or water. In
49 CFR, DOT was established criteria for packaging, labeling, placarding,
shipping paper, and the training and responsibilities of transportation
personnel.
Hazard terminology labeling and placard
IMO
classes and division Vs. DOT
Class 1 Explosive
1.1
mass
explosive
1.2
projection
hazard
1.3
fire
hazard
1.4
no
significant hazard
1.5
very
sensitive substance
Explosive A ; function by detonation
Explosive B ; function by rapid combustion
Explosive C ; manufactured articles containing
Class 2 Gases
2.1 flammable gases
2.2 non-flammable gases
2.3 poison gases
DOT same
Class 3 Flammable liquids
3.1 low flash point < 0 F
3.2 intermediate flash point (0 –37 F)
3.3 high flash point (73- 141 F)
DOT Flammable liquid (flash point <100 F)
Combustible liquid (flash point 100- 200 F)
Class 4 Flammable solid or substances
4.1 flammable solids
4.2 substances liable to spontaneous combustible
4.3 substances which emit flammable gas when wet
DOT Flammable solid
Flammable solid/liquid (pyroforic)
Flammable solid with dangerous when wet label
Class 5 Oxidizing substances
5.1 oxidizing substances
5.2 organic peroxides
DOT same
Class 6 Poisonous or infectious substances
6.1 poisonous substances
6.2 infectious substances
DOT poison B
Etiologic Agent
Class 7 Radiological materials
DOT same
Class 8 Corrosives
DOT same
Class 9 Miscellaneous danger substances
DOT ORM (other regulated materials)
: four sections and different colors on placard are used to classify
potential hazrads. Number (0-4) indicate the severity of hazards.
RED (top); Flammability
4 very flammable gases
3 can be ignited at all normal temp
2 need moderate heat before ignite
1 must be preheated before ignition
0 not burn
BLUE (left); Health
hazard
4 too dangerous need SCBA and CPC
3 extremely dangerous need SCBA no skin contact
2 materials hazardous to health still need SCBA
1slightly hazardous to health may need SCBA
0 expose under fire condition like ordinary combustion
YELLOW (right); Stability or Reactivity
4 explosive at normal temp.
3 explosive but require strong ignition source
2 unstable material rapid release energy and heat but not detonate
1 normal stable but can be excited by temp.
0 stable even under fire
WHITE (bottom); other information
EX, “Oxidizers”
ID
Number has four digits to identify chemical and
emergency responses in DOT guidebook
EX, ID 1831 = sulfuric acid, 1005 = ammonia anhydrous
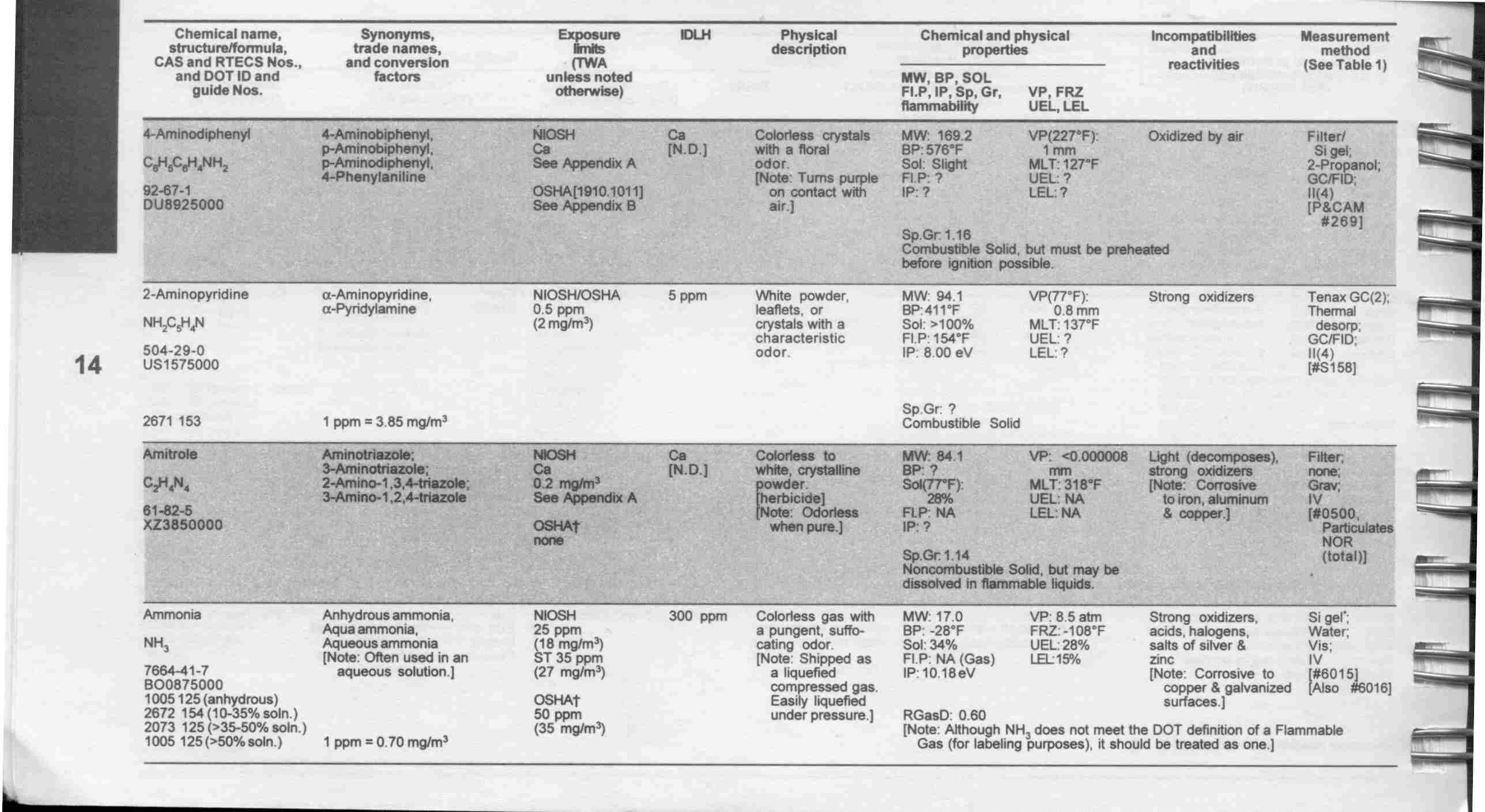
Chemical Trailer

Placard and sign on rail car
Boling
point (BP)
is the temperature at which a liquid began to change to be vapor phase. If the
boiling point is lower than the air temperature around chemical, the chemicals
will boil and become vapor. In some chemicals, ex, Hydrogen chloride (HCl) bp =
-85 C, the chemical is present in gas form at ambient temperature. The leak of
chemical will be seen by the form of toxic fume (slightly yellow gas) and may be
carried by wind.
Vapor
pressure represents
the pressure inside of a closed container by the vapor in the space above the
liquid the container hold. The pressure unit is determined by the mm Hg that
vapor can push. The high vapor pressure chemicals are more likely to breach
their container when heated. Chemicals with high VP are more volatile and more
vapor will present at spill area. Ex, at 75 F, water = 21 mmHg, Ethyl alcohol=
44 mmHg, Chlorine= 4,800 mmHg
Vapor
density is
the mass of vapor divided by the volume filled. VD grater than 1.0 will sink on
the floor. If the leak is a heavy vapor and inflammable, the leak will travel
along the ground to ignition sources. Ex, gasoline VD = 3-4.0 or toluene VD=
2.49
Solubility
and specific gravity are
indicated the status of chemical in the water. Lower solubility can be
easily to separate from the water in the from of the layer or separated part in
water. The specific gravity is referred by comparing of the water at 1.0. The
high specific gravity will sink and the lower specific gravity will float on the
surface of water. For high solubility the chemicals will dissolve in the water
by varying of temperature. The spill control might be very difficult when high
solubility spills are present in the water.
Flash
point is a
minimum liquid temperature at which the vapor will be present above liquid to
ignite. The lower flash point is higher fire hazard. All ignite sources must be
removed from the area near the low flash point chemicals. Ex, gasoline = -45 F,
Ethyl alcohol= 55 F
Explosive
limit range is
reresent the concentration of material that permit the material to burn. LEL
(lower explosive limit) is the lowest ignitable concentration and ULE (upper
explosive limit) is the highest ignitable concentration. The percent or
concentration greater than UEL or lower than LEL will present the range of
non-explosive concentration. Ex, LEL of acetylene gas =3 % and UEL =82%. The
explosive range is 3-82%.
Water-reactivity
materials react
with water to release heat or flammable toxic. If there is a fire in which water
is used as extinguisher, the presence of water can make more danger. Ex,
sulfuric acid leak can generate more heat or potassium and sodium can react with
water producing hydrogen gas.
Oxidizers
The
oxidizers that contain oxygen may release that oxygen as decomposed helping to
sustain a fire. Ex, organic peroxides, acetyl peroxide, peracetic acid, are
considered very dangerous hazards, and also inorganic peroxides like sodium and
potassium peroxide.
Unstable
materials
are those that have a tendency to decompose by themselves. They generate heat or
gas even burnt to a flame or explodes. Some monomer plastics can spontaneously
form polymer causing rupture and explode of container. Ex, PVC monomer, resin,
synthetic rubber, and also include organic peroxides too.
Incompatible
materials
the reaction of some materials can cause heat, violent reaction and toxic fumes.
Ex, (spent caustic + spent acid) =
heat, (asbestos + cleaning solution) = toxic substance, (water + sodium) =
flammable gas
Radioactivity
Radioisotope
chemical can give of energy to knock off electron in molecule or tissue causing
adverse effect. Ex, radon gas, uranium 235, strontium
Mutagens
are the
chemicals that can damage human DNA or any genetic materials in human cells.
Although automatic repair can be occurred by human cells, the results of the may
present changes in DNA or and functions of cells. The changes may lead cancer
cells or some variety of illnesses in human. Ex, Hydroxylamine, Radon gas, or
high intensity of UV
Teratogens
are the chemicals that cause damage in the unborn baby.
Lead is known as a teratogen causing brain damage in offspring.
Neurotoxins
are the
chemicals that can damage brain or sensory or motor nerve communications. Some
organic solvents, for ex, toluene or methyl ethyl ketone, can cause damage in
central nerve system.
Hepatotoxins
are the
chemicals like Hydrogen cyanide, formic acid, or many chlorinated solvents that
can damage liver tissue.
Nephrotoxins
Some workers in assembly plant and metal work can
have more double in the risk of kidneys disease. The damage will not be found
until ¾ of the functions are failed. Arsenic and lead are the examples of
nephrotxins.
Blood
circulation toxins
Ex, CO can form HbCO that is nearly 210 times affinity that oxygen or cytotoxic
hypoxia which blood cells lose ability to utilize the oxygen by the chemicals,
ex, hydrogen sulfide or hydrogen cyanide
The National Research Council’s Committee on Toxicology has published the exposure limits for emergency responders and general public. Emergency Exposure Guidance Limits (EEGLs) and Short-term Public Emergency Guidance Levels (SPEGLs) were published for the protection of people in the community. EEGls are set for protecting the occupational groups such as firefighter, Hazamat responders that typically is younger and healthier than the general public. SPEGLs are set to protect public from effects that might be irreversible or incapacitating. For protecting general population, the Federal Emergency Management Agency (FEMA) lists the following options in order to decrease preferences:
Consult a toxicologist
Use high values among these following:
IDLH/10
TLV-STEL
TLV-TWA
TLV-C
(**for only less than 1 hr operation with no carcinogen**)
Personal Protective Equipment
Chemical
protective clothes and gloves
Chemicals protective clothes are required to protect skin from the chemicals that can absorb and pass through others organ of human body. The selection of CPC has to consider the concentration of chemicals and breakthrough time that protective suit can stay protecting the users during performing the activities. The attack of chemicals to CPC can be classified as permeation, penetration, and degradation. Permeation refers to the process that solid, liquid, or vapor can passes through protective material at molecular level. Penetration refers to bulk movement of chemicals pass through the pore, small flaws, or imperfect seam such as zippers, pinholes or any crack on suit. Degradation refers to the loss of physical resistance to chemical due to chemical reaction. Moreover, the physical wear or abrasion can cause damage to protective suit. The operator has to consider the body heat that generate during the activity and also the radiant heat from some heat sources in operating area in case of fire of work near heat source.
Gloves
and boots are the most protective equipment that contains a lot of contaminants
from the spilled area. Gloves and boots are used to protect arm from both
chemical and physical hazards. Sometimes, selected gloves must cover in both
physical and chemicals two-layers or outer gloves may need for high protection.
·
Site control
Controlled
zones in spilled area
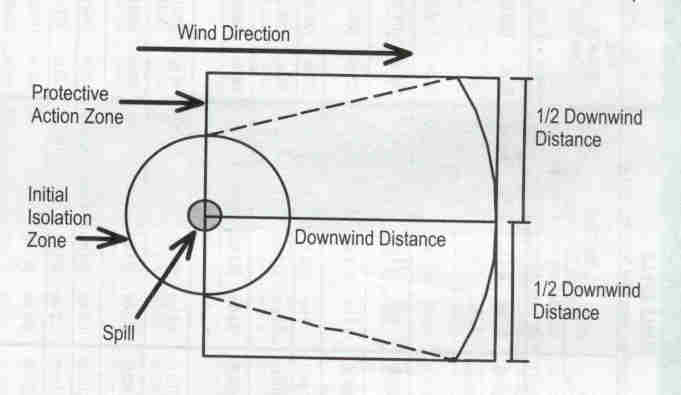
Hot Zone
This location is the area where chemicals hazard was released. This area is the most
dangerous in the site. The hot line is determined based on the risk and hazard
assessment. The PPE are required for operator in emergency response.
Warm Zone (contamination reduction zone)
The warm zone is the area between hot zone and cold zone (uncontaminated area). This area can serve as buffer zone from the spill. The PPE and support equipment for decontamination process are set up in this area. The protective gears are refilled or changed and clean up processes for operator are done in this area.
Cold Zone
The
incident commander post is set up in this area. PPE is not required for the area.
This area provide communication and first aid and medical procedures
·
Confining operation
Confining processes are considered as defensive operations. The selection of appropriate procedures in release control can reduce the spread of chemicals through the public area and reduce among of cost in clean up process. The types of release can be classified in the ways the contaminants can migrate or spread out to the public area. The effective control requires the prediction of the pathways that hazard chemicals will migrate and the factors that support the release, ex, wind, rain, flow direction of current, or shape of earth’ surface.
Land release and control
Diking
involves construction
to confine the migration of spilled hazardous chemicals. Constructing dams by
soil or chemical barrier are required may be in series to stop the release.
Diversion
refers to
the constructions that change or control the pathway of chemicals to protect the
public areas or try to collect the hazardous chemical in the specific place for
cleanup.
Inlet
blockage
requires tight seals at entry point
Water release and control
Heavy insoluble materials
o
Overflow dam
o
Catch basin
Light insoluble materials
o
Barrier or sorbent booms
o
Underflow dam
o
Filter fences
Soluble materials
o
Seal booms
o
Dam
o
Chemical treatment
Air releases and controls
Vapor knockdown by using direct fog
patterns from fire hoses through the chemicals cloud. This method is most
effective in water-soluble materials
Vapor suppression by
using foam blanket to cover toxic vapor. Generally, aqueous film-forming foam
(AFFF) is used to cover on the flammable liquid spills. However, if the
flammable liquid is polar solvent the may be dissolved, reducing foam
effectives.
Containing
processes are considered as offensive operations. The operations are performed
at the source of release. The operators are required extremely protective level
due to the highest hazard potential in this area. Response personnel should not
try to perform containment operation at the point of release before the
procedures and the protective gears have been explored.
Assessment
consideration for selection of containment
In order to identify the procedural option the specific factors related
to the release must be classified. The factors generally can be listed as
follows:
Properties of hazard materials
Physical state; solid, liquid, gas
Physical properties; vapor pressure, density, solubility
Toxicity and flammability; flash point
Reactivity
Characteristic of container
Size and amount containing
Materials the container made of
Characteristic of breach
Size of opening
Shape of opening
Location
Volume and rate of release
Expect duration of release Equipment
required
Wooden plug, Expandable pipe plug, boiler plug, metal screw with gasket,
toggle bolt patches
Adhesives epoxy, plug and patch putty
Sealant and gasket; viton sheet, neoprene sheet, duct tap
Clamp, strap and gasket holder
Valves and fittings Plugging Patching Pump and
vacuum collection Sorbent collection by using sorbent contained in sheet, rolls, or boom Solidification
by solidifier Gelation by using gel-forming chemicals Decontamination line for level A and B Exercise Emergency Plan Levels of exercise Typically,
is used as the introduction for individuals who are in the part of emergency
response. It provides instruction and explanation of overall plan. Tabletop
is the process of sharing the information of each individual. The practice
problems are set for the exercise in decision-making process. It brings people
to form a group in order to exchange the information and provide the best
alternative solution. It is response procedure
to demonstrate the understanding of response plan. The real equipment and PPE
are used like ordinary task. The simulation of emergency action may be performed
to evaluate the effectiveness of emergency plan. Sometime, it is called “hand
–on” activities It offers opportunity for
full implement ERP for specific scenarios. This exercise enhances realism. All
equipment and activities are simulated as the real emergency situation.
The exercise processes